In the ever-evolving world of pharmaceuticals, maintaining a secure and reliable drug supply chain is crucial. Pharmaceutical serialization compliance has emerged as a key factor in ensuring that medications reach patients safely and efficiently. This article explores the ins and outs of serialization compliance, highlighting its importance, challenges, and the future of pharmaceuticals.
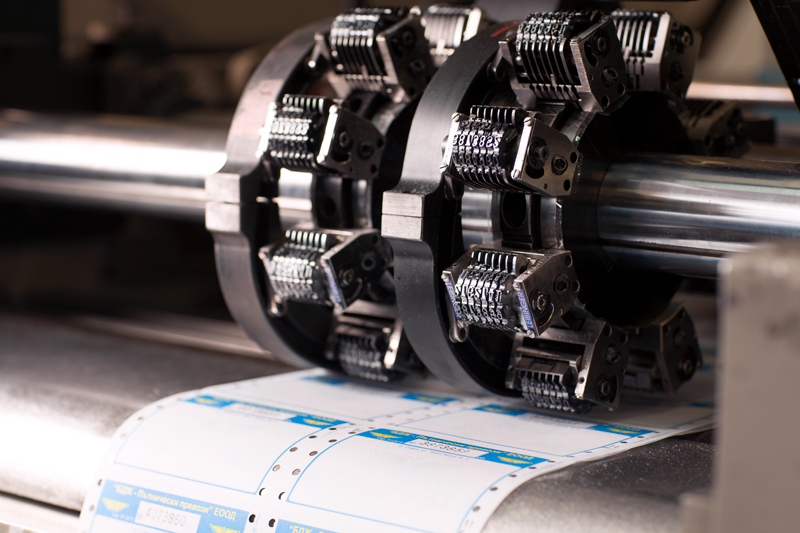
Understanding Serialization in Pharmaceuticals
Serialization is the process of assigning a unique identifier, such as a serial number, to each product. In the pharmaceutical industry, this means every package of medication can be tracked from manufacturing to the end consumer. This unique identifier helps in tracing the origin and journey of the drug, ensuring authenticity and reducing the risk of counterfeit products.
The Importance of Serialization Compliance
Compliance with serialization regulations is crucial for pharmaceutical companies. It not only helps in combating counterfeit drugs but also ensures regulatory adherence. Countries worldwide have implemented serialization requirements to protect patients and maintain trust in the healthcare system. For instance, the United States has the Drug Supply Chain Security Act (DSCSA), while the European Union follows the Falsified Medicines Directive (FMD).
Benefits of Serialization in Pharmaceuticals
The benefits of serialization extend beyond compliance. It enhances supply chain visibility, improves inventory management, and facilitates product recalls. By having a transparent view of the supply chain, companies can quickly identify and address any issues, ensuring uninterrupted drug availability to patients.
Challenges in Pharmaceutical Serialization
While serialization offers numerous advantages, it also presents challenges. Implementing serialization systems requires substantial investment in technology and infrastructure. Companies must also ensure data integrity and security, as any breach could have severe consequences. Additionally, navigating the varying regulations across different countries adds complexity to the compliance process.
The Role of Technology in Serialization
Technology plays a pivotal role in successful serialization. Advanced software solutions enable seamless data management, ensuring accurate tracking and reporting. Leveraging technologies like the Internet of Things (IoT) and smart labels enhances the efficiency of serialization processes. For more insights on IoT and smart labels, visit IoT and Smart Labels.
Future Trends in Serialization
The future of pharmaceutical serialization is promising. With advancements in technology, we can expect more streamlined processes and improved data analytics. The integration of Artificial Intelligence (AI) and machine learning will further enhance supply chain efficiency and security. To stay updated on the latest trends in serialization, check out Anti-Counterfeiting Labels.
Global Serialization Regulations
Understanding the global landscape of serialization regulations is vital for pharmaceutical companies. Each country has its own set of rules, and compliance is not optional. Staying informed about these regulations is essential to avoid penalties and ensure the global distribution of medications.
Serialization in the United States
In the United States, the Drug Supply Chain Security Act (DSCSA) outlines serialization requirements. It mandates that all prescription drugs must have a unique identifier for traceability. Companies must also implement electronic systems to capture and maintain transaction information.
Serialization in Europe
Europe follows the Falsified Medicines Directive (FMD), which requires serialization for prescription drugs. The directive aims to prevent counterfeit medicines from entering the supply chain. It mandates the use of a 2D data matrix code and a unique identifier on each package.
Implementing Serialization Systems
Implementing a serialization system requires careful planning and execution. Companies must evaluate their current infrastructure, invest in the right technology, and train their workforce. Collaboration with technology providers and regulatory experts is crucial for a successful implementation.
Steps to Achieve Serialization Compliance
To achieve serialization compliance, companies should follow a structured approach. This includes conducting a gap analysis, developing a detailed implementation plan, and ensuring seamless integration with existing systems. Regular audits and updates are essential to maintain compliance and adapt to changing regulations.
The Impact of Serialization on Patients
Serialization has a direct impact on patient safety. By enabling traceability, it ensures that patients receive genuine and safe medications. In case of recalls, serialized products can be quickly identified and removed from the market, minimizing harm to patients.
Serialization and Counterfeit Prevention
Counterfeit drugs pose a significant threat to patient safety. Serialization acts as a powerful deterrent against counterfeiting. By making it difficult for counterfeiters to replicate unique identifiers, serialization helps maintain the integrity of the drug supply chain.
The Future of Pharmaceutical Serialization
The future of pharmaceutical serialization is bright. As technology evolves, serialization processes will become more efficient and cost-effective. Companies that embrace serialization today will be better positioned to meet future regulatory requirements and ensure the safety of their products.
Conclusion
Pharmaceutical serialization compliance is a critical component of ensuring a safe and reliable drug supply chain. By embracing serialization, pharmaceutical companies can protect patient safety, maintain regulatory compliance, and enhance supply chain efficiency. As the industry continues to evolve, serialization will remain a cornerstone of pharmaceutical operations.

FAQs
What is pharmaceutical serialization compliance?
Pharmaceutical serialization compliance involves assigning a unique identifier to each drug package for traceability and regulatory adherence.
Why is serialization important in pharmaceuticals?
Serialization ensures the authenticity of drugs, prevents counterfeiting, and enhances supply chain transparency.
What are the challenges of serialization?
Challenges include high implementation costs, data security concerns, and navigating complex global regulations.
This article contains affiliate links. We may earn a commission at no extra cost to you.







